You’re reading the web version of our email briefing on health policy, science and medical news. Sign up to get it next week.
Hi Healthwatchers, 👩🏻⚕️👨🏾⚕️👩⚕️
Ready to dive into this week’s biggest health stories?
More U.S. items than usual made it into this edition, but each carries real weight for Canada. From a major lawsuit affecting health records in both countries, to climate-driven blood shortages that could easily hit us next, the ripple effects are easy to see.

Proposed changes to Alberta’s Bill of Rights could allow health workers to opt out of workplace vaccine requirements, ending measures in place since the 1980s.
Why it's important: Alberta hospitals have long required workers to receive vaccines for hepatitis B, varicella, and influenza among other things. The amendments risk undermining these protections.
If the Bill of Rights is amended as-described by Danielle Smith, vaccine uptake among health workers is likely to drop, increasing the risk of preventable disease outbreaks and further destabilizing the province’s healthcare workforce.
Read more…
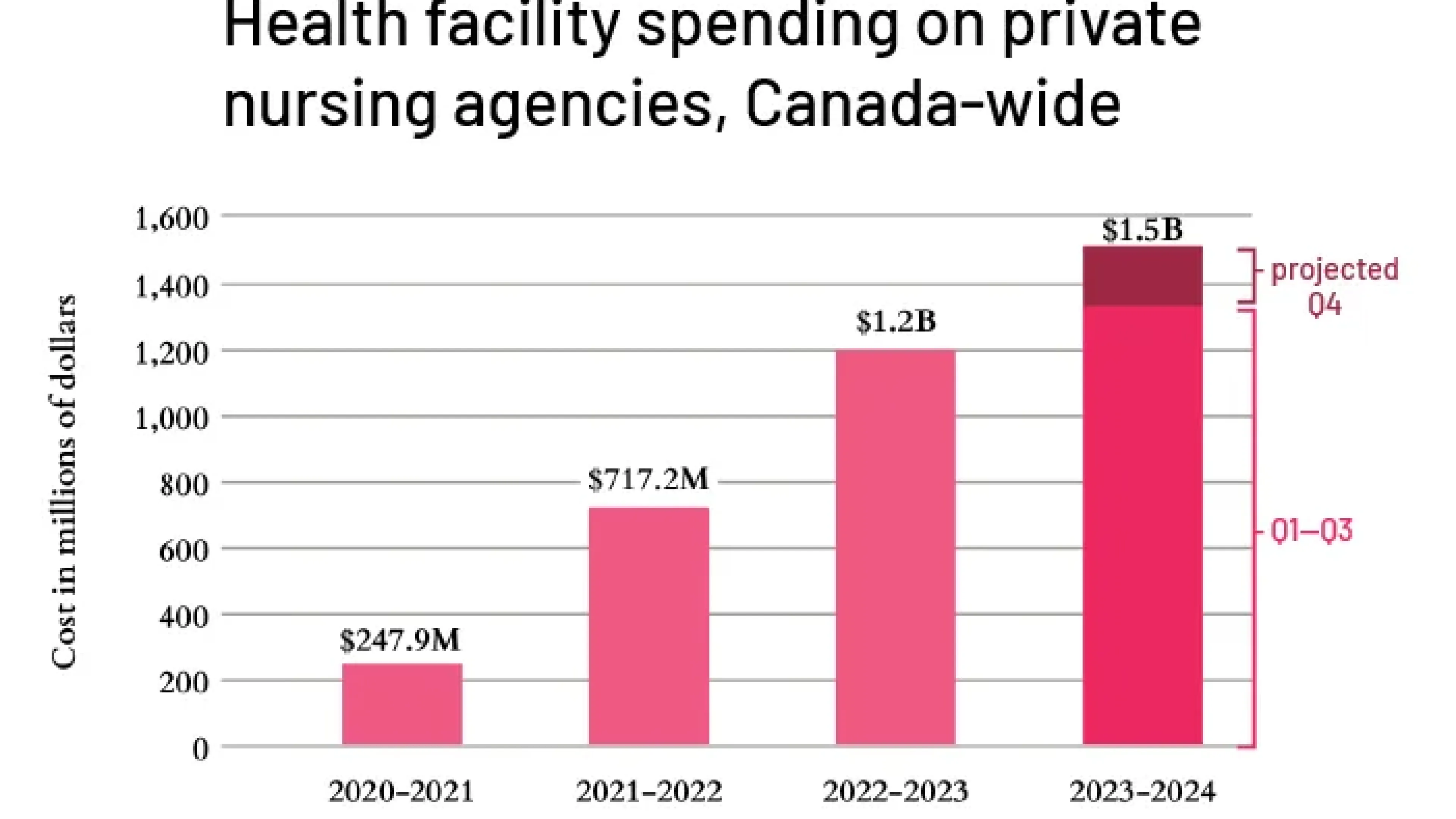
Hospitals and nursing homes spent over $1.5 billion on private nurses last year, a six-fold increase since 2020, according to a new report.
Why it's important: This is a vicious cycle. As reliance increases on costly agency nurses, more nurses take jobs with staffing agencies. Institutions then struggle to find and keep permanent staff, leading to a loss of institutional memory and more errors.
The $1.5 billion spent last year could have been used to improve nurse retention and stabilize the workforce, rather than fueling the expansion of agencies. A P.E.I. hospital currently has more agency nurses than staff nurses in its critical care program.
Read more…

Health data startup Particle Health has sued Epic Systems, arguing the electronic health records company uses its control over patient data to stifle competition.
Why it's important: It’s a U.S. story, but Epic is an increasingly powerful player in Canada’s EMR software space, giving it significant power in the health data market.
Particle says that Epic blocks smaller rival software platforms from accessing essential medical records, effectively blocking competition. This lawsuit has the potential to either accelerate or seriously deflate the push for interoperability in healthcare.
Read more…

Ontario's Bill 7 allows hospitals to place discharged patients in long-term care homes without consent, imposing a $400 daily fine if they refuse to leave.
Why it's important: Critics argue the law coerces vulnerable patients, undermining autonomy and worsening health outcomes. Patients can be placed up to 150km away.
While the law’s intention is to alleviate bed shortages, the number of alternate level of care patients taking up hospital beds has increased by 30% since it passed. As other provinces consider similar changes, this ruling will have national implications.
Read more…

Heatwaves and storms are contributing to blood shortages, as people stay away from donation centers during severe weather, according to a senior HHS official.
Why it's important: Blood shortages severely impact hospitals' capacity to perform surgeries and treat patients. Extreme climate events are making them worse.
While the immediate solution is more donations, the long-term impact of climate disruption on health infrastructure, including blood banks, needs urgent attention.
Read more…
The FDA approved donanemab despite concerns over its safety and efficacy, and advisors’ financial ties to the drug's manufacturer.
Why it's important: The conflicts raise serious questions about the integrity of the FDA’s approval process. The drug shows minimal cognitive benefit alongside risks like brain swelling and deaths.
With safety data missing, donanemab remains on the U.S. market while its risks are studied until 2037. It’s currently under review for approval in Canada.
Read more…
Thanks for reading today’s edition of The Weekly Dose!
We'll be back next week with more health updates and news that matters.
What did you think of this week’s stories?
Nick Tsergas
Health News Editor
Canada Healthwatch
[email protected] | canadahealthwatch.ca
Stay informed.
On the most important developments in Canadian health.
Get Canada’s essential briefing on health policy, science, and system change. Get Briefing