The importance of evidence in the 2024 breast cancer screening guideline
We live in a world where new medical evidence is published daily on health conditions like heart disease, cancer, long COVID, autoimmune disorders and more. We are inundated with data from randomized controlled trials (RCTs), observational studies and other types of research. The methods and quality of these studies range from well-designed to poor quality, making it challenging to summarize and interpret.
The synthesis of evidence for national clinical guidelines is critical for primary health providers and their patients. Clinicians seek easy-to-understand, trustworthy guidance and tools that they can share with their patients to help with individual care decisions. Like the broad range of medical issues that occur in primary care, guidance is needed on diverse topics such as screening for various cancers, thyroid dysfunction, chronic health conditions, as well as other interventions such as preventing tobacco use and falls.
The Canadian Task Force on Preventive Health Care (Task Force) fulfills this need. It is a long-standing, independent, national panel of health care professionals with expertise in primary care, evaluating evidence, and the development of preventive care guidelines. The guidelines they produce are evidence-based recommendations for family physicians, nurse practitioners and other primary care providers.
Members volunteer their time and expertise and are vetted to ensure neutrality in assessing evidence and developing recommendations; they do not have ties to industry or specialty organizations, nor financial conflicts of interest. Its core members are family physicians, nurse practitioners, specialists and people with expertise in comprehensive evidence review. Together, they provide a balanced benefits and harms perspective on key health care topics. They follow international best practices to review and synthesize evidence to develop relevant, high quality clinical practice guidelines for primary care providers.
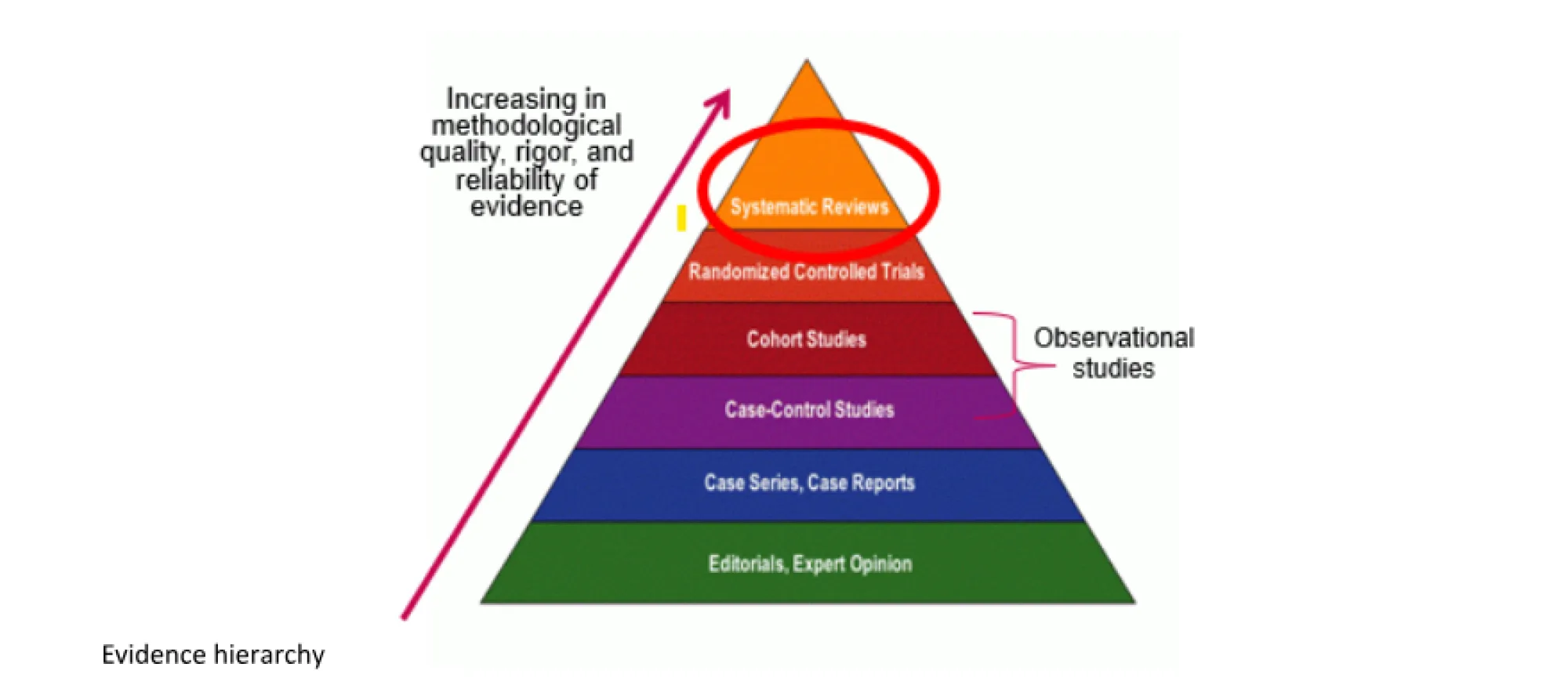
Breast cancer screening guideline update release in 2024
Breast cancer is a disease that touches many Canadians. We all want to find ways to reduce the burden of this disease and improve outcomes for the many people and their families who have endured suffering and tragedy resulting from it.
The Task Force is currently conducting a comprehensive evidence review to update its 2018 breast cancer screening recommendations and will release its new recommendations in the spring of 2024. The Task Force is assessing available evidence, both past and current, including recent observational trials, RCTs and modelling. The Task Force’s role is to summarize the best available evidence and make recommendations that consider the benefits and harms of screening, as well as patient values and preferences. This ensures primary care providers have access to high quality guidance and help patients make informed choices on preventive care, such as breast cancer screening.
For each guideline, the Task Force relies on subject matter experts and patient input. The breast cancer screening guideline update includes four such experts (a clinical oncologist, a radiation oncologist, a surgical oncologist and a radiologist), and three patient partners. They also engaged the TF-PAN (Task Force Public Advisors Network) to better understand what patients want to know.
Both the current 2018 guideline and the updated 2024 draft recommendations that will soon be released are for women (people assigned female at birth) with no personal or extensive family history of breast cancer, genetic risks or symptoms of breast cancer like a breast lump.
The current guideline emphasizes discussing benefits and harms of screening between patients and health care providers so that patients can choose if they want to be screened. This approach recognizes differences in the relative value that individuals place on the potential benefits and harms of screening. It is important to note that people aged 40-74 years of age who want breast cancer screening should have access to a mammogram.
The updated 2024 recommendations will be accompanied by tools to support discussions between health professionals and their patients to enable the person to make an informed decision.
What did the Task Force consider for the update?
As part of its comprehensive evidence review, the Task Force asked questions such as what are the benefits and harms of screening across age groups? What is the importance of the harms and benefits of screening for people? Does screening reduce breast cancer deaths, reduce advanced disease (with less aggressive treatments), and lead to better quality of life as beneficial outcomes? It also sought to understand whether the benefits and harms of screening differ by population characteristics (e.g., age, breast density, race and ethnicity, socioeconomic status, availability of mammography screening and family history).
The Task Force works closely with research teams from across Canada, including the University of Alberta, University of Ottawa, and the University of Manitoba and Institute for Health Economics (Edmonton). Based on the questions asked by the Task Force, the research teams are systematically reviewing and analyzing the scientific evidence for the guideline. They will then provide the Task Force with a comprehensive evidence summary on which to base recommendations. Both RCTs and observational studies are included in the evidence review — RCTs are the internationally recognized gold standard for understanding the effects of healthcare interventions. However, for breast cancer screening, most of the RCTs are older. To ensure recent evidence was examined, the Task Force expanded the evidence review to include recent observational trials as well as modelling. They are considering all these study types along with data from Statistics Canada and other sources to make sure they have the best and most recent evidence. The evidence review is very broad and assessed thousands of articles for potential inclusion. In addition, the evidence team re-analyzed and included the work of the 2023 United States Preventive Services Task Force to inform this update. This ensures that updated recommendations are based on the best evidence available.
For this review, equity is a major consideration and available screening evidence is being assessed for different populations, including age, race and ethnicity and breast density, as well as in adults with sex-specific breast tissue.
Opportunity for input
This spring, the Task Force will release draft recommendations for stakeholder and public input. The final guidelines will be available later this year, including trustworthy and easy-to understand patient and health provider tools to ensure Canadians have the best evidence to make informed decisions about their health.
The Canadian Task Force on Preventive Health Care operates independently, with funding by the Public Health Agency of Canada (PHAC). Its valued work aims to improve Canadians' overall health and well-being by addressing key issues and advising on effective preventive strategies.

Dr. Guylène Thériault
Dr. Guylène Thériault is the Chair of the Canadian Task Force on Preventive Health Care

Dr. Donna L. Reynolds
Dr. Donna L. Reynolds is a Family Physician, Assistant Professor of Public Health and Preventive Medicine Specialist